Blog
Degradation of Xylene in Gas Phase by Ozone and UV Light

Abstract :The degradation of xylene (p2、m2、o2xylene) by ultraviolet light and ozone was investigated in different conditions. The degradation reactions of xylene in air and nitrogen were compared. The intermediate products of xylene in the photo-degradation reaction in air and the mechanism were discussed. The experiment results indicated that the photo2 degradation rate of p2 xylene was more than the others , and the intermediate products were detected by direct GCPFID analysis under the same conditions.
According to some statistics, modern people spend more than 80% of their time staying indoors and the time is much longer for the aged people and the infants. Therefore, the vast majority of the air that people breathe comes from indoor air and its quality has direct effects on people’s health.
According to the Canadian Health Organization survey, it is shown that 68% of human diseases are related to indoor air pollution currently. After having done a lot of research, the National Environmental Protection Agency proposed a theory called Sick Building Syndrome , for short SBS. It pointed that symptoms of SBS have a direct link with indoor air pollution. Benzene series is an important indoor pollutant, of which xylene has a stimulating effect on the eyes and upper respiratory tract, and can cause fatigue, head pain, blurred consciousness, central nervous system inhibition.
The toxicity of xylene is renal toxicity, neurotoxicity and embryo toxicity. Due to its stable structure, it is difficult to degrade by general method. Ultraviolet (UV) has been widely used in medical, food and small scale drinking water disinfection and volatile degradation of organic matter due to its bactericidal effect. Lots of researches have been done to study the removal of benzene and toluene by UV by researchers at home and abroad, while the study on the degradation of xylene in gas phase is rare. In this paper, the UV and ozone in the gas phase of xylene in the gas phase were studied by using the quartz ultraviolet germicidal lamp as the light source. For future research, it has accumulated some experimental data.
1 Experiment
1.1 Main experimental instrument and reagent
GC2MS: QP22010 type gas chromatography mass spectrometry
Quartz ultraviolet sterilization lamp
Ozone generator
Ozone analyzer of Model 49C
P-Xylene, m-Xylene and o-Xylene
1.2 Experimental facility
Place UV lamp for 20W germicidal lamp (main wavelength 254nm),in the axis position of glass cylinder (long 520mm, diameter 98mm) and then use aluminum foil and PTFE as sealing materials.
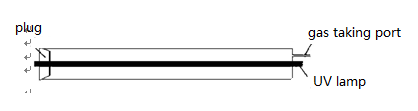
1.3 Experiment contents
1.3.1 Experimental conditions
Flexible Fused Silica Capillary Column: DB25 ( 30m ×0. 25mm , 0. 25μm)
Gas Carrier: He (99%)
Column Pressure: 0. 05MPa
Split ratio: 300: 1
Vaporization room temperature: 70℃
Column temperature: 45℃
Test room temperature: 150
Ionization voltage: 70ev
Ion source temperature: 300 ℃
Interface temperature: 28℃
Scanning mass range: 30 ~ 550AMV
1.3.2 Experimental Procedures
a. When using the ultraviolet light to degrade organic matter, seal the reaction container, inject the quantitive reactant by the sample injector, and a certain concentration of the experimental gas is prepared. Set the sample to be mixed evenly, turn on the UV lamp, and sampling every 20min to measure the concentration of the substance.
b. Degradation of organic compound with ozone
Firstly, produce a certain amount of ozone by ozone generators. Then use ozone analyzer to monitor the concentration of ozone. Then seal, inlet, and stewing. After the adsorption equilibrium was reached, the concentration of the substance was measured every 20min.
C. When using ozone and UV light at the same time, just follow step b. After reaching adsorption equilibrium, then turn on the UV lamp. Changes in the concentration of ozone, the initial concentration of reactants and other conditions repeat the above operation
2 Result and Discussion
2.1 Ozone produced under ultraviolet light 's irradiation in different conditions
Use ozone analyzer to monitor change of ozone concentration over time under ultraviolet light’s irradiation. The result shows that the ozone concentration produced in in a reactor filled with oxygen was significantly greater than that of the indoor air. This is because under UV light irradiation, oxygen generates photochemical reaction: 3O2 + H, 2O3 v. UV lamp produces highly oxidizing ozone under air irradiation, which is beneficial for the removal of organic matter from the air.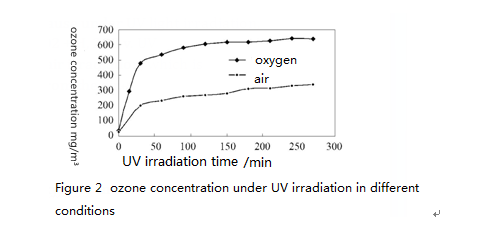
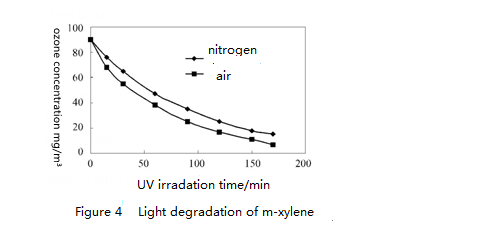
2.2 Degradation of o-xylene, m-xylene and p-xylene in different reaction systems
Figure 3 to 5 show light degradation of initial concentration 90 mg/m³of o-xylene, m-xylene and p-xylene under different conditions. Only under the UV light irradiation, degradation rate in the air of the same concentration of o-xylene, m-xylene and p-xylene is greater than the degradation rate in nitrogen. This is because that the air generates ozone under UV light irradiation and ozone accelerates degradation rate of o-xylene, m-xylene and p-xylene. Under the combined effect of ozone and UV light for about three hours, the degradation rate of o-xylene, m-xylene and p-xylene all reached over 80%.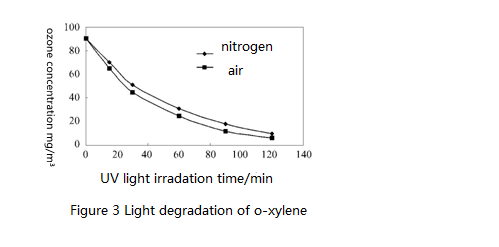
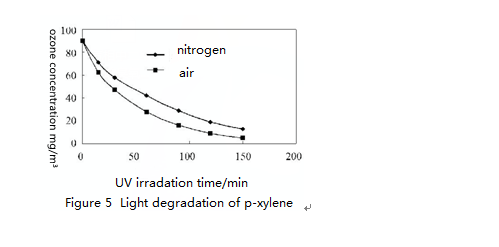
After dynamic analysis of experiment data of o-xylene, m-xylene and p-xylene light degradation of 20min, 40min, 60min and 80min of different reaction systems, the results showed that the reaction was in accordance with the first order kinetics. The degradation rate of o-xylene, m-xylene and p-xylene are shown in Table 1
Table 1 Reaction rate constant of xylene in air and nitrogen
| Reagent | p-xylene | o-xylene | m-xylene |
| Rate Constant | 1.38 | 1.53 | 2.03 |
As can be seen from the table 1, under the same conditions, the degradation rate of the o-xylene is the largest due to its steric hindrance and poor chemical stability. Therefore, it is easy to be oxidized and degraded under the light condition.
2.3 The removal rate of o-xylene, m-xylene and p-xylene under different reaction conditions
Figure 6 ~ 8 shows respectively the removal rate of o-xylene, m-xylene and p-xylene at the same concentration (90mgPm3) under the combined effect of ozone (concentration 712 ug/m³) and UV light. Obviously, only under the effect of ozone, o-xylene, m-xylene and p-xylene removal rate increases with time and degradation rate reached about 50% 1 hour later. By contrast, removal rate of o-xylene, m-xylene and p-xylene increased greatly and can be up to 85% one hour later. Under UV light irradiation, when increasing ozone concentration to 2026 ug/m³, the removal rate of o-xylene, m-xylene and p-xylene of the same concentration all reached 90% after twenty minutes reaction.
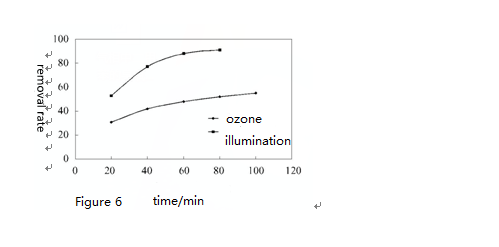
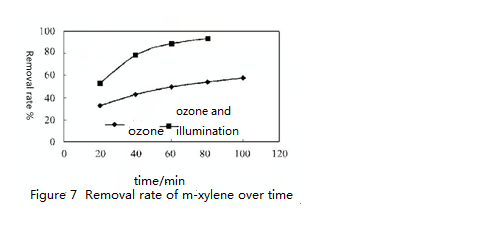
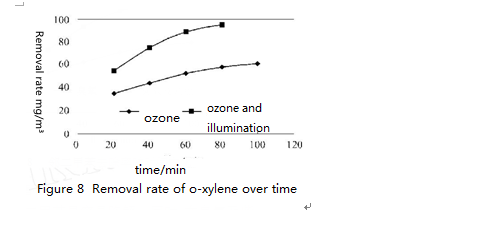
It can also be seen that the o-xylene’s reaction rate is the largest under the same condition, which shows that it is the most easily to degrade. At the same time, under the combined effect of ozone and ultraviolet lamp irradiation, the degradation efficiency of o-xylene, m-xylene and p-xylene is greater than that in UV lamp or ozone alone. This is because that under the combined effect of ozone and UV light irradiation, degradation of o-xylene, m-xylene and p-xylene is completed through two ways. On one hand, it is degraded by the occurrence of chemical bonds facture after absorbing photons of certain energy. On the other hand, under the strong oxidation of atomic oxygen and hydroxyl radical, o-xylene, m-xylene and p-xylene is degraded. Under the combined effect of ozone and UV irradiation, their degradation reaction rate increased greatly.
2.4 The degradation products of o-xylene, m-xylene and p-xylene and its reaction mechanism
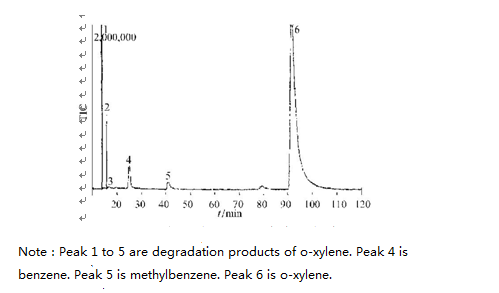
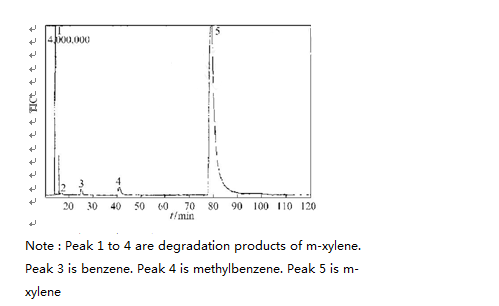
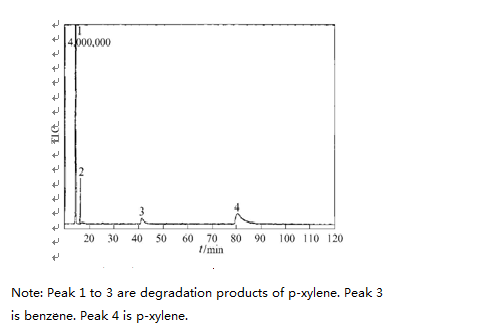
During the course of this experiment, the degradation of xylene has intermediate products. In figure nine, we can see GC2MS spectra of intermediate product of o-xylene, m-xylene and p-xylene after 120mins UV light irradiation.
From Figure 9, it shows that the toluene and benzene were generated in the degradation process. The reaction formula is as follows: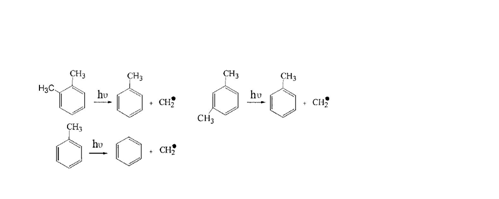
As for the generation of benzene during the process of light degradation of xylene, the reaction formula may be as follow:
Other reaction products need to be further studied.
Richard A considered that under the conditions of NO2, no and O2, the main intermediate products of xylene degradation are methyl benzaldehyde and methyl benzene methyl alcohol. It is obvious that there will be different intermediate products under different conditions.
3 Conclusion
Under ultraviolet light, the photochemical reaction of oxygen generates ozone, so the ozone generated in the ultraviolet light in air is greater than the amount of ozone produced in nitrogen. Strong oxidation property of ozone makes the light degradation rate of o-xylene, m-xylene and p-xylene in the air far greater than that in nitrogen. Under the combined effect of ozone and ultraviolet lamp irradiation, the degradation efficiency of o-xylene, m-xylene and p-xylene is greater than that in UV lamp or ozone alone. This is because that o-xylene, m-xylene and p-xylene not only can absorb photons energy to break chemical bond, but also can be degraded under the action of oxygen atom and hydroxyl radical. Due to its large steric hindrance and poor stability, o-xylene is much easier to be degraded for the same condition. The experiment showed that intermediate products are produced during the degradation process.





